Carpal Tunnel Release System Market to Grow 72.5% by 2035 Across APAC, Europe, U.S., and Saudi Arabia
Global carpal tunnel release system market set to reach USD 1.15 billion by 2035, driven by minimally invasive surgery demand.
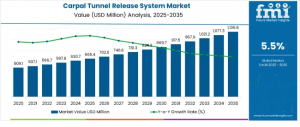
DE, UNITED STATES, November 7, 2025 /EINPresswire.com/ -- The global Carpal Tunnel Release System Market is poised for steady expansion, projected to increase from USD 665.4 million in 2025 to USD 1,147.7 million by 2035, marking a 72.5% growth over the forecast period. The market will expand at a CAGR of 5.5%, supported by increased prevalence of repetitive strain injuries, rising awareness of surgical treatment options, and accelerating demand for minimally invasive procedures across key regions including North America, Europe, Asia-Pacific, and Saudi Arabia.
Carpal tunnel syndrome continues to impact millions of individuals globally, particularly among computer-based professionals, manufacturing workers, and aging populations. With the increasing focus on workplace ergonomics and early intervention, healthcare systems are prioritizing procedures that improve function, reduce chronic pain, and support faster recovery.
Explore trends before investing — request a sample report today!:- https://www.futuremarketinsights.com/reports/sample/rep-gb-5965
Market Dynamics and Growth Outlook
Between 2025 and 2030, the market is projected to expand by USD 212.1 million, accounting for 44% of the decade’s overall growth. This period will be defined by rising preference for minimally invasive and outpatient surgical options—particularly in ambulatory surgical centers. Between 2030 and 2035, the market will add another USD 270.2 million, representing 56% of projected growth, supported by the integration of robotic-assisted systems, improved visualization technologies, and optimized endoscopic procedures.
The Open Carpal Tunnel Release (CTR) System remains the leading product category, accounting for 62% market share in 2025. Its well-established clinical reliability, surgical familiarity among specialists, and lower procedural complexity continue to position it as the dominant choice. However, the Endoscopic CTR segment is gaining traction due to advantages such as reduced scarring, faster healing, and better cosmetic outcomes—particularly among working-age patients seeking minimal downtime.
End User and Healthcare Setting Trends
Hospitals are set to account for 60% of global demand in 2025, supported by strong surgical infrastructure, comprehensive reimbursement systems, and concentration of specialized orthopedic and hand surgery expertise. Meanwhile, Ambulatory Surgical Centers (ASCs) are expanding rapidly, driven by reduced wait times, cost-efficient care models, and rising patient demand for same-day surgical recovery options.
Regional Market Highlights
• United States (CAGR 5.2%): Leading adoption of advanced surgical visualization systems and expanding ASC-based procedures.
• Europe (CAGR 5.6%): Germany and France drive demand through clinical excellence and investment in surgical innovation.
• APAC (India CAGR 6.5%, China CAGR 6.1%): Growth fueled by expanding healthcare access and increased diagnosis of repetitive strain-related conditions.
• Saudi Arabia and GCC: Government healthcare modernization efforts continue to boost adoption of standardized orthopedic surgical solutions.
Competitive Landscape
The market remains moderately consolidated, led by established medical device companies including Stryker Corporation, Smith & Nephew plc, CONMED Corporation, Arthrex Inc., and Integra LifeSciences. Key strategic focus areas include surgeon training, endoscopic system refinement, device miniaturization, and integration of robotic assistance.
Emerging players such as Sonex Health LLC and A.M. Surgical Inc. are gaining prominence through specialized minimally invasive instruments and competitive price positioning.
Click Here to Purchase the Report:- https://www.futuremarketinsights.com/checkout/5965
Key Drivers
• Increased incidence of work-related musculoskeletal disorders
• Growth in minimally invasive and outpatient surgical procedures
• Advancements in visualization and surgical precision systems
• Expanded reimbursement for early-stage nerve decompression procedures
Challenges
• Requirement for surgeon training in advanced techniques
• Budget constraints in developing healthcare markets
• Competition from non-surgical intervention therapies
Latest Therapeutic Device Reports:-
Joint Reconstruction Devices Market
https://www.futuremarketinsights.com/reports/joint-reconstruction-devices-market
Orthopedic Prosthetics Market
https://www.futuremarketinsights.com/reports/orthopaedic-prosthetics-market
Laparoscopic Device Market
https://www.futuremarketinsights.com/reports/laparoscopic-devices-market
Why Choose FMI Empowering Decisions that Drive Real-World Outcomes:- https://www.futuremarketinsights.com/why-fmi s
About Future Market Insights (FMI)
Future Market Insights, Inc. (ESOMAR certified, recipient of the Stevie Award, and a member of the Greater New York Chamber of Commerce) offers profound insights into the driving factors that are boosting demand in the market. FMI stands as the leading global provider of market intelligence, advisory services, consulting, and events for the Packaging, Food and Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals markets. With a vast team of over 400 analystsworldwide, FMI provides global, regional, and local expertise on diverse domains and industry trends across more than 110 countries.
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware – 19713, USA
T: +1-347-918-3531
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube
Sudip Saha
Future Market Insights Inc.
+1 347-918-3531
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.